Carbon
Carbon can exist in various "formations", but its chemical properties will always be the same! This phenomenon is called allotropy.
There are many allotropes of carbon which can be categorized into two categories.
These categories are Amorphous and Crystalline.
Graphite

Graphite has a layered structure in which the distance between two layers is much more than the distance between two C-atoms.
This makes it very easy for layers to slide upon each other, thus giving a slippery texture.
That is why, graphite can be used as a lubricant.
Graphite is a crystalline allotrope.
Diamond
Diamond is a very expensive allotrope of carbon.
It shines brightly because of the light reflected from it.
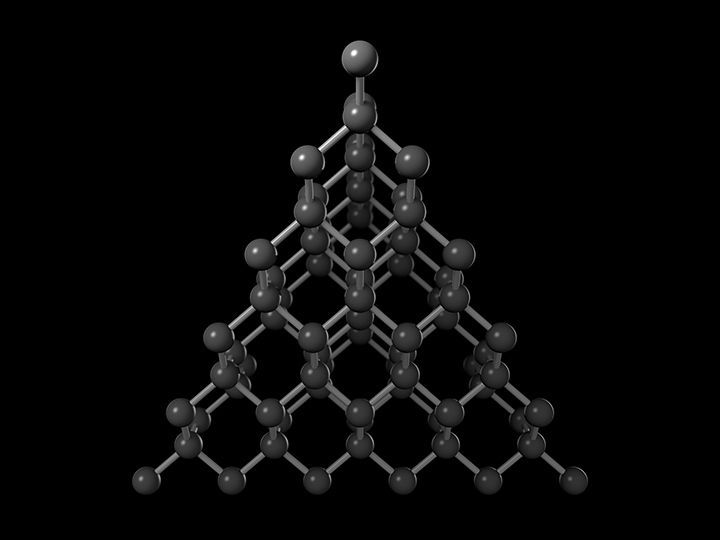
It is tetrahedral in structure.
The bonds are very strong which makes it the hardest substance in the whole world.
Buckminster Fullerene
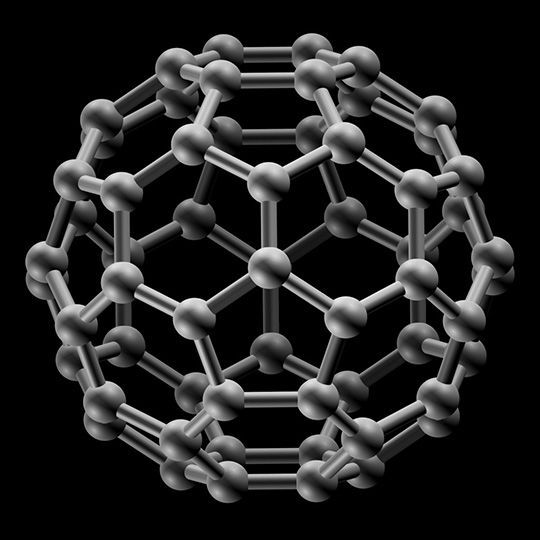
This is a superconductor allotrope of Carbon.
It looks like a football.