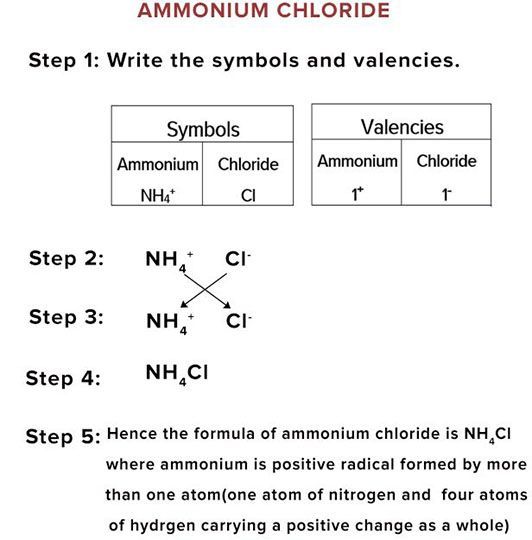
Writing the Chemical Formula
To write the chemical formula (of a compound) the following information should be available:
1. Symbols of the elements or the radicals
that constitute the compound.
2. Valencies (combining capacity) of the
elements or the radicals.
The method applied for writing the
chemical formula is called criss-cross
method.
Criss-cross Method
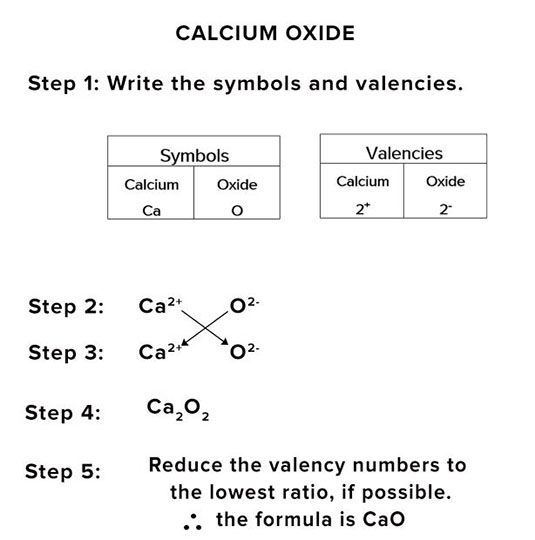
When the valencies of the combining atoms are equal.
e.g. Ca2+ and O2-.
Calcium oxide.
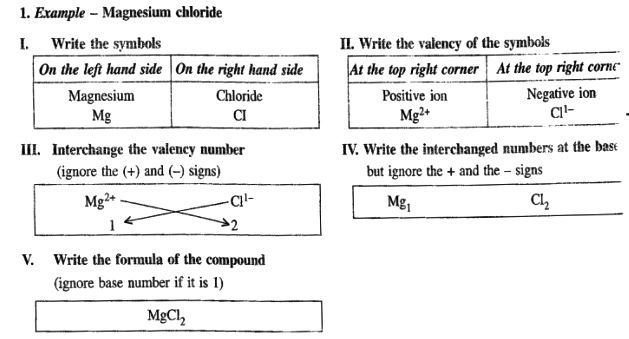
When the valency of the cation is greater than the anion.
e.g. Mg2+ and Cl-
Magnesium Chloride.
When the cation has more than one atoms in it.
It is treated as one molecule and the factor is multiplied with the whole molecule.