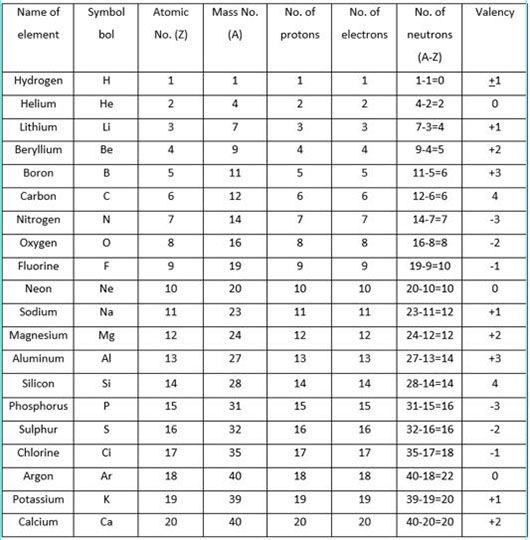
Valency
Valency is the combining capacity of an atom of an element with atoms of other elements to form molecules.
Variable Valency
Some elements exhibit more than one valency. They're said to have variable valency.
Examples : Iron, copper, tin, lead, sulphur, phosphorus.
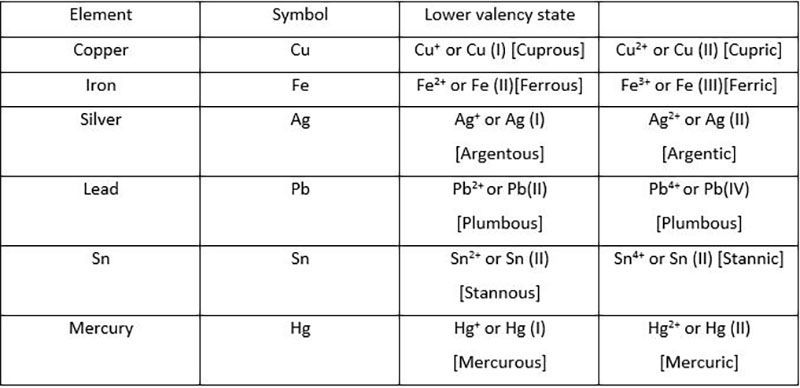
In the case of the metals exhibiting
variable valency, we represent the lower
valency by adding the suffix 'ous' to the
name of the metal; to represent the
higher valency the suffix 'ic' is attached
to the name of the metal.
For example , the metal iron has
valencies +2 and +3. For the lower
valency (+2) we write ferrous (Fe2
+) and
for the higher valency (+3) we write
ferric (Fe3
+).

In the modern method the variable
valency of the element is represented by
Roman numbers.
Thus ferrous ion is represented. as Fe (II) and ferric ion as
Fe (III).
The advantage of the modern convention
is that neither the name of the element
nor its symbol changes.
In the case of a non-metallic atom
the number of the other types of
atoms attached to it determines its
valency.
Phosphorus has valencies 3
and 5. With chlorine it forms two
compounds, PCl3 and PCl5. Therefore the
molecule of phosphorus trichloride, which
has three chlorine atoms in it, has the
lower valency (3), and the molecule of
phosphorus pentachloride, which has five
chlorine atoms in it, has the higher valency
(5) for phosphorus atom.